Formaldehyde-free Wet-State Preservation for Modern Paints and Coatings
The trend toward more environmentally-sensitive paints and coatings is well established worldwide. Whether driven by new regulatory constraints imposed by national, regional or local air quality organizations or by a desire to carry a quality certification from such organizations as Green SealTM 1 or GreenGuard® 2, the die is cast. While initial efforts focused on volatile organic compound (VOC) content of the final coating, the next phase is going well beyond VOC reduction. This current effort is geared to removal of other specific objectionable materials from paint formulations, including formaldehyde. Part of the desire to remove these materials is based on technical merit; but part is based on emotional reactions to perceived hazards. The distinction really makes no difference; making technical arguments to counter a well ingrained perception is generally a waste of time.
For the preservative supplier and user, reduction of VOC content attributable to preservative use has been on-going for several years. While the potential amount of VOC carryover from the use of wet-state preservatives is quite small (due to the low use rate of these preservatives), it became a near-universal supplier goal to help the paint formulator meets his goals for VOC reduction in their final product. It is now possible to provide adequate preservation to coatings without carrying any measurable preservative-based VOC into the coating formulation. Costs of employing such programs are generally modest, some 1.5 – 2 times as much as the older preservation programs being replaced. This cost increase is generally acceptable for a premium coating, but less palatable for an economy grade coating. Therefore, the latter paint grades will continue with older preservation technology for some time since these older preservative programs still carry much less than 5 g/L of VOC into a coating as a worst case. This is acceptable for a general grade paint that will be trying to achieve 150 –250 g VOC/L; but possibly not for a premium coating trying to achieve 50 g VOC/L.
The next hurdle for premium coatings is to carry a low formaldehyde content. While zero formaldehyde is impossible (formaldehyde being a natural constituent of our ecosystem), considerable effort will be made to reduce the deliberate or known introduction of formaldehyde into premium paints and coatings, particularly those that might seek certification from organizations like Green Seal or countries like China. In this effort, preservative providers have a definitive goal to try to minimize formaldehyde introduction into coatings as a consequence of preservatives use.
Historically, the preservation industry has featured first mercury, then later organic compounds know as formaldehyde adducts. While the molecular structure and chemical properties of formaldehyde adducts vary, in activity they are more alike than different. These products feature strong antibacterial performance, resiliency to bacterial challenge, and long lasting protection. And they are relatively inexpensive. Under alkaline conditions, the generation of free headspace formaldehyde from these products is very low, well within any OSHA-required labeling limits. However, when coatings containing these chemistries dry on the wall, then formaldehyde does off-gas and thus there is a legitimate concern for such emissions at this point.
The actual potential exposure to formaldehyde emission can be, and has been, quantified. For example, emission chamber experiments have been performed to mimic a freshly painted room as it goes through the drying process. Tightly controlled experiments have been performed in an environmental chamber modeled to be equivalent to a 9 foot by 12 foot room with 8 foot ceilings (with one door and one window) and covered with drywall material. It was shown that the emission of formaldehyde from drying paints containing a normal dose of a model formaldehyde adduct did not liberate enough formaldehyde to approach the OSHA workspace exposure limit for any air exchange rate greater than 0.2 room changes/hour 3. However, rather than fight a technical battle with non-technical end users and regulators, most coating producers are, or will, opt to spend their resources to eliminate known influxes of formaldehyde – at least for their premium paint lines. Organizations such as GreenSeal are eager to help this process along and have proposed ‘standards’.
While not intending to produce a treatise on formaldehyde, some common concepts about formaldehyde and formaldehyde adducts must be discussed to better understand the science inherent in the goal of achieving a low formaldehyde content. A basic requirement for achieving low formaldehyde content is a formaldehyde measurement method that is accurate, reliable, reproducible, sensitive, and widely accepted. Furthermore, concepts of what is meant by ‘free’, ‘total’, and ‘theoretical’ formaldehyde also must be discussed and agreed upon. Finally, a basic requirement is an understanding that the measured formaldehyde content of a coating depends on how you measure it, including under what conditions this measurement is performed. Measurement by another method or even the same method under different conditions will yield a completely different result.
Under alkaline aqueous conditions, formaldehyde is in dynamic equilibrium between different loosely bound forms. Strictly speaking there is very little free formaldehyde present under these conditions; it is always bound with something and as such is not very volatile (as easily demonstrated by headspace analysis). The role of a FA is to drag the equilibrium to favor itself as the formaldehyde-bound form and minimize other forms of formaldehyde since these forms are counterproductive to sustained bacterial protection. Because of this equilibrium, trying to measure ‘free’ formaldehyde depends heavily on the test conditions. The analysis is always trying to measure a moving target as any attempt to measure ‘free’ formaldehyde changes the equilibrium and thus changes the ‘free’ formaldehyde present. In the final analysis, free formaldehyde as measured by such gentle analysis as exemplified by the German ‘Blue Angel’ methodology (VdL03)4 is not only highly variable; it will ultimately under-represent the amount of formaldehyde potentially present.
What is needed is a total formaldehyde method that can assess the total formaldehyde which can be liberated from the coating during the drying process. To do this, harsher reaction conditions are required. However, harsh test conditions results in materials that may not liberate formaldehyde during normal coating dry to do so under these more rigorous test conditions. An over-estimate of the actual formaldehyde content that might be liberated is the result. Many of these analytical methods are known and have been used for years. Historically they have been time consuming and prone to large measurement errors. However, there are some new methods that feature a derivativization reaction followed by a HPLC finish that show great promise, albeit still tending to overestimate the actual formaldehyde that might be liberated from the coating during drying (because of rigorous initial reaction conditions needed).
In the interim, there are organizations that have promulgated methods to measure formaldehyde content of paint and coatings. One example is the Chinese GB 18582-2008 method5. This method has an Appendix C that carries the unfortunate title of “Determination of Content of Free Formaldehyde “. Unfortunate, because the method does not measure ‘free’ formaldehyde but rather something between “free” and total formaldehyde as discussed above. In practice, the method is prone to some large inter-laboratory variations (see graph) and under the best of conditions has an expected error of ±20%. But it may become the de facto standard as no other organization or country has leapt so prominently into the forefront.
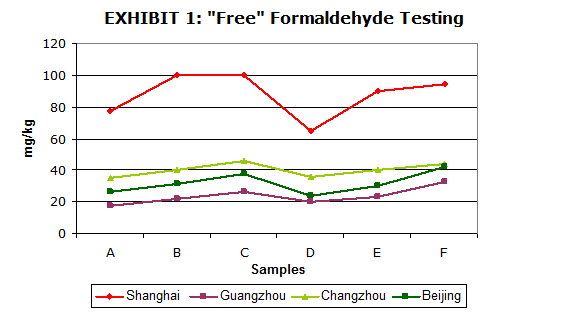 Exhibit 1:
Exhibit 1: “Free” formaldehyde as measured by four Chinese laboratories authorized to perform
Chinese Standard GB 18582-2008. Six different paint samples were sent separately to each of these authorized test labs for ‘round robin’ testing.
With this background in hand, it is possible to propose preservative chemistries that should not, of themselves, trigger any formaldehyde concerns when used in coatings. This is not to say that the coatings do not face challenges in regards to formaldehyde testing; preservatives are only one potential entry point for formaldehyde, or products that can degrade to formaldehyde under the test conditions. What are some of these proposed chemistries? The foremost one is going to be 1,2-benzisothiazolin-3-one (BIT) which is well known in the industry. Others are the classic isothiazalone products, bronopol, and mixtures of these materials. There are a few other potential candidates, but they are not well known or well enough established as wet-state preservatives in their own right to make predictions as to their utility going forward.
On the plus side, these highlighted wet-state preservatives are not only well known in the industry, they are also VOC-free. On the negative side, none of these materials will be the total solution to providing effective, reliable wet-state preservation. In practice, it does not take long to find situations in which each of these chemistries will fail dismally as a preservative. Some times the failure is based on chemical incompatibilities such as a coating pH, temperature, or the presence of reducing compounds. Other times the failure is based on the lack of disinfecting ‘power’ in the preservative itself – it works too slow to stifle out-of-control situations, its mode of action is too weak for the problem organisms, or it cannot be used legally at high enough dose rates. In our experience, it is the rare coating in which any of the proposed preservatives can be totally effective as the sole addition. And there are applications where none of these preservatives has any hope of being effective.
As an example, examine Exhibit 2 which illustrates some of these issues. Exhibit 2 shows a typical two tier challenge study in which a test coating is treated with a preservative ladder (BIT in this case) and then the survival of bacteria is monitored over time. During each tier, survival is measure after 24, 48, and 166 hours of exposure. Successful completion of two tiers of challenge testing is widely regarded as indicative that the coating is well protected and suitable for market (this result indicates about one year’s shelf stability – depending on subsequent challenge events, storage conditions, etc). The observed patterns from the graph are quite common when testing BIT alone in many coatings. The relative bacterial survival at the end of the testing tends to be somewhat insensitive to BIT concentration until some matrix determined dose rate threshold is reached. At higher dose rates, the peak bacterial populations after a challenge event are muted; but the ending bacterial population is about the same (lack of dose response). In this case, even if the system is wildly overdosed with BIT, the end point remains essentially the same (and as shown here the end point is not passing the criteria commonly set for success). This type of a result is not universal, but it is by no means rare in working with BIT. Additional work is ongoing to investigate what is causing this ‘plateau’ effect in many coatings. At least two mechanisms come readily to mind – bacterial coverage gaps in BIT performance (which are known) or the presence of bacterial spores (which are insensitive to preservatives entirely). Since this effect is so pronounced only with BIT products, the former is the most likely explanation of the two. But there may be other explanations as well.
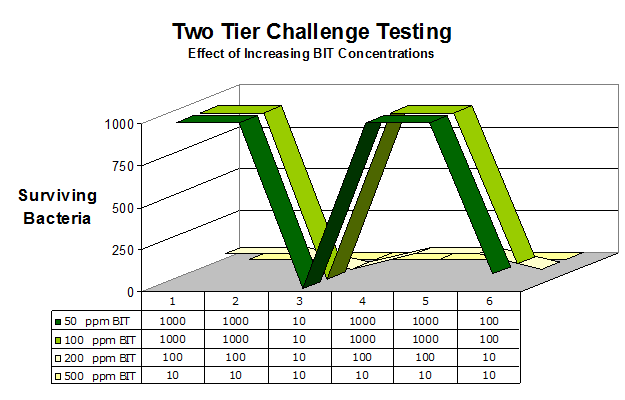 Exhibit 2
Exhibit 2. Two tier challenge test results in a commercial coating. The coating treated with ladders of BIT was challenged twice with a standard mixture of bacterial. Survivors were assayed after 24, 48 hours and then 7 days after each challenge. The survivors are plotted for each assay time. A dose and matrix determined plateau effect is often seen with BIT products.
So what to do? The other possible alternative preservatives suggested earlier also have recognized shortcomings. Isothiazolin-based preservatives often do not have the necessary longevity in coatings with a pH greater than 8, especially when stored at elevated temperatures. The presence of any reducing agents left over from the polymer manufacturing process will also dramatically shorten the half-life of these products. Likewise, bronopol is adversely affected by these conditions.
We would suggest two routes forward. One is improved strategies for wet-state preservation at the final packaging step, the other is to improve plant hygiene to deliver the coating to the fill head in a condition much easier to protect by the available preservatives. Both strategies ultimately may be required to succeed in all cases.
Improved Preservation at Packaging:
Significant work has been performed to show that combinations of the available zero-VOC, zero-formaldehyde preservatives can work effectively together to deliver a final preservation package of significant power. After studying numerous mixing strategies, the best strategy seems to be to ‘base load’ with a BIT-containing product and then supplement with one or more of the other more effective, but shorter-lived, preservatives. The complimentary preservatives essentially cover the short term inadequacies of BIT as well as the well known Pseudomonas gap in its range of coverage. Interestingly, even with the known short-life span of the supplemental preservatives in a typical coating, it can be readily shown that the final preservation provide by such a combination is not only effective but robust and able to withstand repeat bacterial challenges (See Exhibit 3).
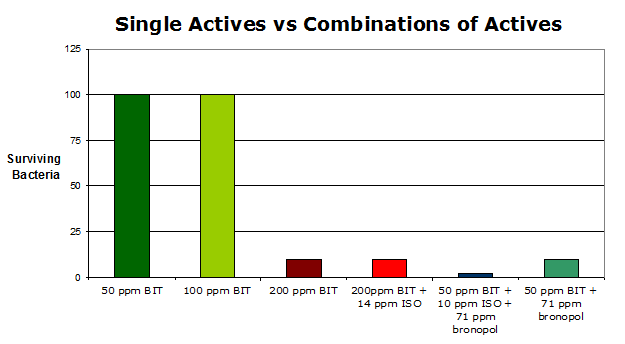 Exhibit 3:
Exhibit 3: Final results of two tier challenge testing in a commercial coating. The treated test coating was challenged twice with a strong bacterial mix. Survivors were assayed after 24, 48 hours and then 7 days after each challenge. The dose rate showing an absence of survivors on the seventh day after the second challenge is reported. The value of using a combination of actives is evident.
Improved Plant Hygiene
Volumes have been written on plant hygiene and its importance in the production of high quality coatings and paint. While beyond the scope of this paper, improved plant hygiene allows a microbiologically cleaner product to be presented for final preservation and packaging. In the context of this paper, preservation programs applied as a part of a plant hygiene program can be constructed in such a manner as to provide longer term benefits to the final product by acting as supplements to the final preservation program. Whether used as an overspray to resin tank headspaces, for wash water clean up, or as slurry or clay preservatives, careful preservative selection and optimization can insure carry over effects to other areas in the plant. In this context, preservatives that would not qualify as acceptable preservatives for the final protection of a ‘green’ coating could well be the key to allowing those deemed ‘acceptable’ preservatives to succeed at the end of the day. 5
Examination of preservation data presented here shows that one of the key preservatives for producing green coatings, especially while keeping BIT additions at reasonable concentrations is bronopol. Unfortunately, when subjected to the rigorous analytical testing procedures required to test for formaldehyde in a coating, bronopol can release small amounts of formaldehyde. While bronopol is not a formaldehyde adduct and does not act through the release of formaldehyde, there will remain some theoretical potential for some formaldehyde release. Fortunately, in actual practice the formaldehyde release in a coating by bronopol, even when evaluated under vigorous analytical conditions, is low (about 7% of theoretical). In most cases, it is difficult to separate bronopol’s contribution from the contribution of other coating additives (background formaldehyde). Exhibit 4 shows an experiment in which bronopol’s contribution to total formaldehyde content was measurable (see Exhibit 4).
Exhibit 4: Total Formaldehyde Content
|
SPECIMEN
|
HCHO
|
|
Latex Coating A
|
12 ppm
|
|
+ 71
ppm bronopol + 450 ppm BIT
|
16 ppm
|
|
+ 71
ppm bronopol + 675 ppm BIT
|
16 ppm
|
|
Latex Coating B
|
12 ppm
|
|
+ 71
ppm bronopol + 450 ppm BIT
|
16 ppm
|
|
+ 71
ppm bronopol + 675 ppm BIT
|
16 ppm
|
|
Latex Coating C
|
13 ppm
|
|
+ 71
ppm bronopol + 450 ppm BIT
|
17 ppm
|
|
+ 71
ppm bronopol + 675 ppm BIT
|
17 ppm
|
|
Latex Coating D
|
13 ppm
|
|
+ 71
ppm bronopol + 450 ppm BIT
|
17 ppm
|
|
+ 71
ppm bronopol + 675 ppm BIT
|
17 ppm
|
Exhibit 4: HPLC determination of total formaldehyde content in latex paints, with and without bronopol addition as part of a multi-active approach. Method: Derivatization after acid hydrolysis processing conditions.
Conclusions
It will be possible to adequately protect some coatings by using formaldehyde and VOC free preservatives simply by substitution of chemistries. On paper the most logical substitution will be with BIT since it shares fewer of the drawbacks of other substitute formaldehyde-free actives. The amount of active required will be considerably more than was needed under previous preservative programs. Treating costs will be higher. In perhaps the majority of cases, a preservative program featuring multiple actives will be required as BIT has some performance drawbacks of its own. Whether the multiple actives are added into the final product or are added at several different application points during the manufacturing process will be application specific. Bronopol is a valuable active that can be used effectively even under tight formaldehyde restrictions. Even with its well known pH and temperature limitations, voluminous data exist to show that, in combination, it does provide benefits to a long-lasting preservation process. Only emotional and non-technical considerations block its judicious use. Furthermore, even the classic formaldehyde adducts can be used to treat raw materials and for plant hygiene programs as long as careful attention is paid to application rates and application points. Such judicious use will not result in unreasonable amounts of formaldehyde being present in the final product (data not shown). Finally, the paint manufacturer should consider which ‘rating’ organization to submit green paints. Some rating organizations base their ratings on “formula checking”; others require some actual performance testing. The latter is likely to give the manufacturer more options since formulary examination leads to unnecessarily conservative, non-technical judgments.
References
- www.greenseal.org
- www.greenguard.org
- Formaldehyde Emissions: Separating Myth From Reality. Izzy Colon and Shib Mookherjea, Paint and Coatings Industry, September 1997.
- Verband der Lackindustrie e. V. , VdL-RL 03, Richtlinie zur Bestimmung der Formaldehydkonzentration in wasserverdünnbaren Dispersions-farben und verwandten Produkten.
- Indoor Decorating And Refurbishing Materials--Limit Of Harmful Substances Of Interior Architectural Coating, GB 18582-2008, National Standards of People’s Republic of China, Implemented on 2008-10-01.
- How Microbiocides Will Contribute To Further Substantial Reduction Of Emissions From Coatings. Wolfgang Lindner and Gary Horacek, JCT Coatings Tech, February 2009.